Biological 3D printing (Bioprinting) marks a revolution in the field of personalized medicine, with the potential to transform the discipline. The technology enables the precise production of implants, tissues, and even whole organs, tailored to the individual patient – from corneas for the eyes to skin grafts and new hearts.
Advertisement
The chronic shortage of organs for transplantation and the difficulty of donor-to-recipient tissue matching remain central challenges in medicine. In recent years, innovative technologies have been bringing us closer to the long-sought goal of patient-specific “spare parts.” For example, three-dimensional (3D) printing—a process in which materials are deposited layer by layer to create precise 3D structures—offers a promising solution to these challenges. This technology enables the production of implants, prostheses, and even living tissues [1] that meet specific mechanical requirements and are tailored to the patient, helping to overcome limitations such as immune rejection or anatomical mismatch.
One of the earliest and most established medical applications of 3D printing is the production of customized prostheses and implants. For prostheses—external devices that replace or supplement missing body parts—3D scans of the patient’s body can be used to fabricate highly accurate devices in both size and design. Organizations such as Enabling the Future [2] manufacture affordable prosthetic hands that can be customized for size and shape. For implants placed inside the body, such as cranial or facial implants, biocompatible materials like titanium or specialty polymers are employed to ensure the implant integrates well and avoids triggering an immune response. Printing allows a precise anatomical fit, contributing to both medical success and an aesthetic outcome.
Whereas the design of prostheses and implants focuses on anatomical fit and mechanical strength, the major challenge in 3D printing of living tissues for clinical use—printed from human cells and biological scaffolds—is maintaining cell viability and integrating the tissue into the body. These requirements demand complex control of printing conditions, cellular nutrition, and vascular integration.
A leading example is the work of the Israeli biotechnology company Precise Bio, which is currently establishing a production line for 3D-printed corneas for human clinical trials at Sheba Medical Center [3]. Corneal blindness is a disease in which the cornea thins and changes shape from dome-like to conical, preventing light rays from properly focusing in the eye. This disease is a leading cause of vision loss worldwide and often requires corneal transplantation. The shortage of donor corneas has spurred the development of 3D-printed artificial corneas. The company uses 3D printing to produce implants composed of densely packed human endothelial cells (the cells that line the inside of blood vessels) supported by a human collagen-based scaffold [4]. During printing, collagen is shaped into structures that provide structural support and guide the formation of 3D tissues from the cells that seeded on top of the collagen [1,5]. The human collagen scaffold is designed to be only a few microns thick, enabling easy manipulation during surgery. In the cornea-fabrication process, corneal endothelial cells (CECs) are isolated from a donor cornea and expanded under laboratory conditions until they divide into a sufficient number of cells. These cells are used to print a single-cell layer on top of the collagen scaffold, ensuring uniform distribution and high cell density—both essential for successful graft uptake. During surgery, the printed implant is loaded into a glass syringe and inserted into the patient’s eye through a small incision [6].
The company has already conducted a successful pre-clinical study in rabbits, but to advance to human implantation trials it must meet the U.S. Food and Drug Administration (FDA) regulatory requirements. It must demonstrate that the laboratory-produced tissue functions identically to natural human tissue—including its transparency, mechanical properties, and long-term cell stability. The choice to start with corneal printing stems from the cornea’s unique characteristics: it requires no patient-specific matching because it rarely triggers an immune response. Nevertheless, the company plans to expand its development to other fields such as orthopedics and cardiology and hopes eventually to reach an even more ambitious goal: printing an entire kidney from the patient’s own cells, eliminating the need for anti-rejection drugs after transplantation.
3D printing of more complex tissues, such as skin and cardiac muscle, is also rapidly advancing. In dermatology, bioprinting technologies allow the production of skin grafts for treating burns and chronic wounds. The technology enables patient-specific customization of the tissue, potentially reducing rejection risk and improving healing. Research indicates the possibility of producing skin that includes complex structures such as hair follicles and blood vessels, bringing widespread clinical use closer [7].
Heart transplantation remains the primary treatment for late-stage heart failure, but donor shortages and risk of rejection, infection, and other complications limit its effectiveness. Bioprinting heart tissues derived from the patient’s own cells offers a potential solution that bypasses immune-compatibility issues and organ scarcity. Induced pluripotent stem cells (iPSCs)—adult cells reprogrammed to an embryonic-like state can potentially serve as an unlimited source of any cell type [8]—enable the creation of functional heart tissues that could treat heart failure and congenital defects. However, technological hurdles remain, such as the challenges of printing thick tissues with a sufficiently high cell density [9].
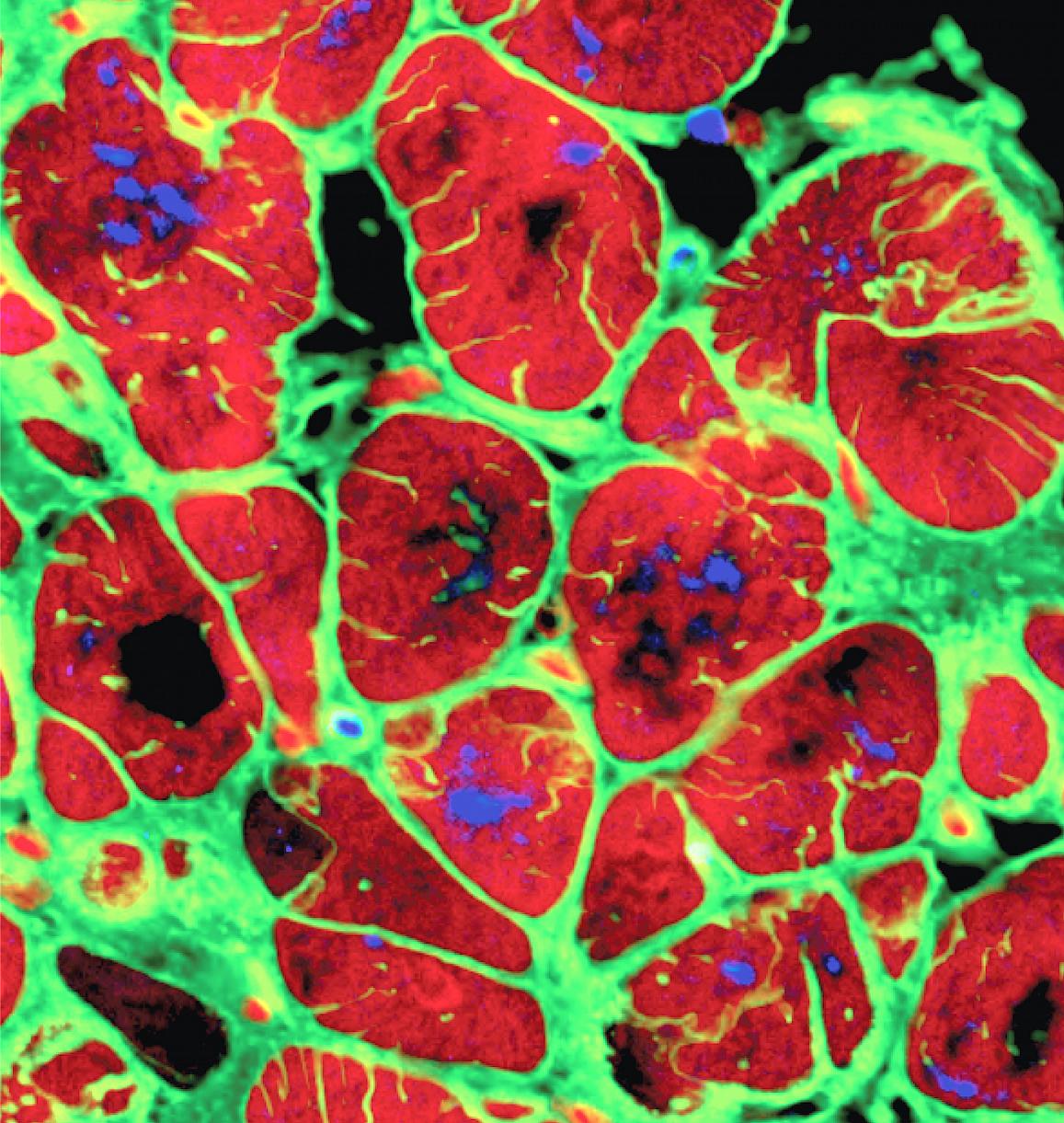
Cardiomyocytes derived from induced pluripotent stem cells (iPSCs) | Columbia University via National Institute of Biomedical Imaging and Bioengineering (NIBIB)
Significant challenges persist, including high costs, complex regulation, the need to improve the compatibility of printed tissues, and the challenges of printing complex tissues composed of multiple cell types and vascular networks. The printing process often cannot produce highly detailed 3D structures at high resolution. Therefore, the future of bioprinting depends on refining printing techniques and selecting and formulating suitable biological materials for building tissues and organs. A multidisciplinary approach combining materials engineering, bioengineering, and biology [10] will lead us to a future where 3D printing enables the production of whole organs for transplantation, fully tailored to the patient.
Hebrew editing: Smadar Raban
English editing: Elee Shimshoni
References:
- Printing a small, patient-matched heart at Tel Aviv University
- Enabling the Future website
- Article in “The Marker” about Precise Bio
- About the collagen protein
- Video showing heart printing
- Article on 3D-printed corneas
- Review article on 3D printing of skin
- About stem cells
- Review article on 3D-printing developments in the cardio-vascular field
- Review article on challenges in tissue printing







