Like underwater sculptures, corals build a calcareous skeleton that forms their reefs – one of the richest and most diverse environments in nature. Although they start as a tiny polyp, they are responsible for constructing gigantic structures visible even from space. So how do they do it? Through biomineralization – a precise biological process that combines specialized cells that secrete ions and proteins. Confronted with the threats of climate change and ocean acidification, corals exhibit an impressive capacity for adaptation.
Advertisement
So, what exactly is biomineralization? It is the remarkable process by which living organisms produce minerals, essentially acting as “biological builders”. This process is ubiquitous. Clams create shells, sea urchins build spines, chickens produce eggshells, and humans construct bones and teeth. And that is only the tip of the iceberg. Currently, about 60 different minerals produced by a wide variety of organisms are known[1]. Biomineralization unfolds through a highly regulated process. Specialized cells secrete ions and proteins into a well-defined space, directing crystal formation with great precision. Strong structures then arise, tailored to each organism’s physical and environmental needs. Each species has its own unique “recipe,” featuring a distinct mineral composition, crystal shape, and guiding proteins that steer the process with engineer-like accuracy.
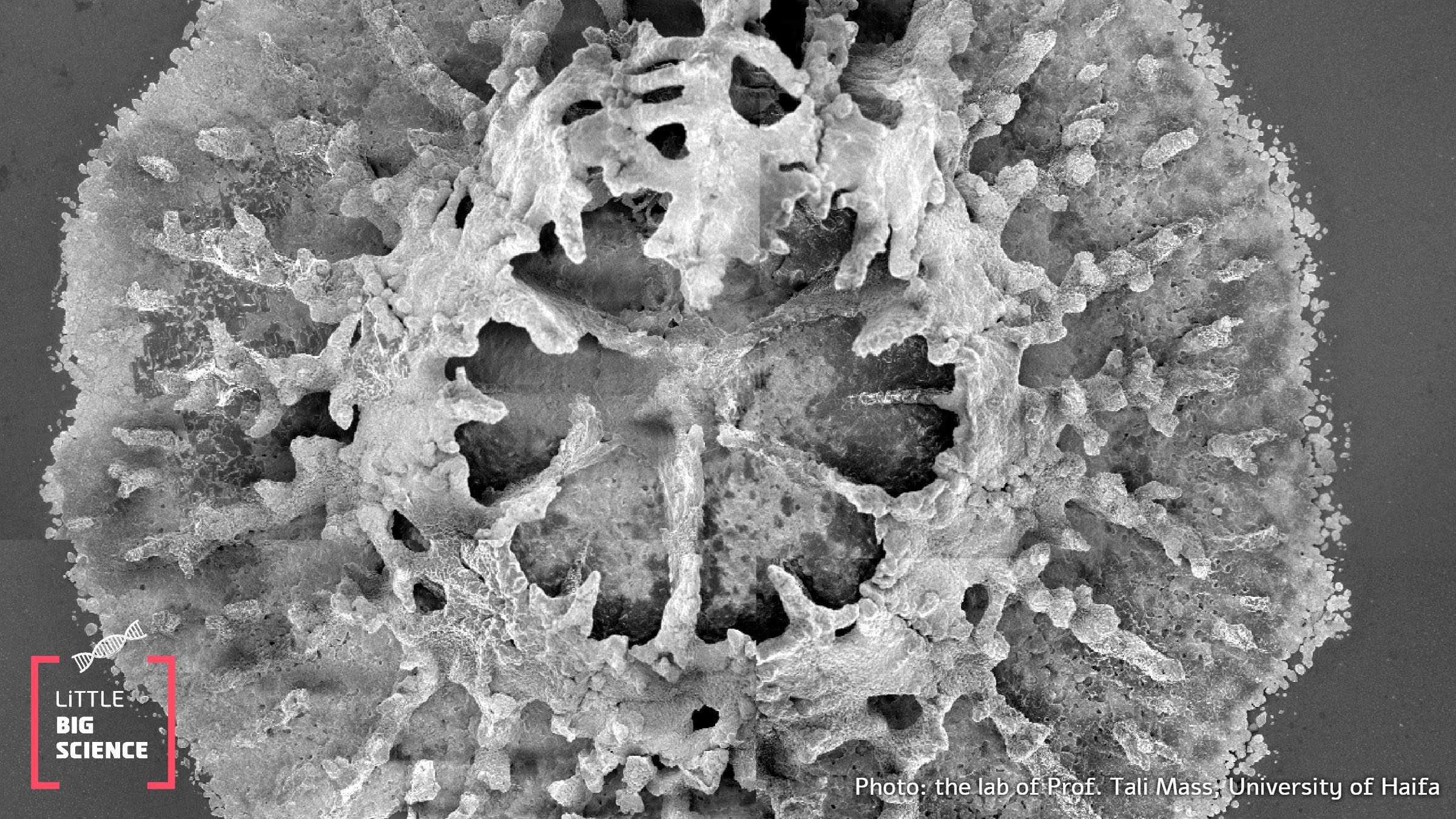
Of all the organisms that perform biomineralization, corals are among the most striking and fascinating examples. It all begins with a polyp, a small flower-like creature that forms the basic unit of a coral reef. At the interface between the polyp’s body and the substrate, special cells called calicoblasts create a sealed cavity known as the “calcifying space”. This cavity forms an isolated pocket where crystallization begins. Calicoblasts pump ions - in this case calcium (Ca²+) and carbonate (CO₃²-), both naturally abundant in seawater—into the enclosed space to initiate mineralization. The combination of these ions produces the inorganic compound calcium carbonate (CaCO₃), the primary mineral used to build skeletons. Similar to how carbon atoms that can be arranged as graphite or diamond, calcium carbonate can be organized in different forms, depending on its atomic arrangement. In corals, it crystallizes as aragonite, a crystal particularly suited for underwater skeletons due to its strength, stability, and structural precision. However, although calcium and carbonate are plentiful in seawater, they do not spontaneously crystallize into calcium carbonate. Crystallization requires precise chemical and physical conditions such as sufficient ion concentration, suitable acidity, temperature, and pressure. Corals create these precise conditions inside the skeletal deposition space.
However, inorganic chemistry alone is insufficient. Calicoblasts also secrete organic substances, such as proteins, that guide crystallization. These proteins control the size, orientation, and the spatial arrangement of crystals, giving the skeleton its precise and unique architecture. These proteins act as biological scaffolds. They determine the position, shape, and the order of crystal formation. Every new polyp follows the same exact rules: it creates the sealed cavity, secretes proteins and ions, and contributes to the growing skeleton. Thus, a continuous, coordinated structure emerges, with each polyp adding to the coral reef's construction, brick by brick, in a marvelous collaboration with thousands of identical individuals [2].
But then acidity enters the picture and disrupts the process. Since the industrial revolution, humans have released vast amounts of carbon dioxide (CO₂) into the atmosphere, mainly by burning fuels. Roughly one-third of this CO₂ is absorbed by the oceans, where it reacts with water to form carbonic acid. This process increases the acidity (lowers the pH) of seawater, a process called ocean acidification [3]. When seawater becomes more acidic, hydrogen ions react with carbonate ions (CO₃²-) and convert them into bicarbonate (HCO₃-), thus reducing the availability of carbonate for building skeletons. In short, the biological scaffolding is still there, and the calicoblasts are still secreting proteins, but the chemical “bricks,” the minerals, are missing. Under these conditions, corals struggle to build and maintain their skeletons. The skeleton becomes thinner and less dense, containing more protein relative to minerals, and therefore weakening the structure. Over time the skeleton becomes vulnerable to erosion, predation, and breakage, endangering the entire reef [4].
Despite these challenges, corals are resilient organisms. They have survived millions of years of change, including mass extinctions and extreme climate shifts. Their ability to adapt, build, and renew is truly impressive. However, the skeletal-building process, with all its biological and chemical aspects, remains largely unknown. Scientists continue to study reefs and make new discoveries about this process, yet much work remains to be done.
To secure a future for reefs and ensure that corals continue constructing these underwater wonders for generations to come, we must protect the oceans. One way to do so is by developing smart conservation programs based on a deep understanding of biomineralization. Just as corals continue to build, repair, and connect even under the harsh conditions, we must do the same. Even when it’s difficult, we should strive to connect, repair, and support one another—and always leave room for hope.
Main image: Scanning electron microscope image showing a primary polyp after settlement—the beginning of a reef | Photo: Prof. Tali Mass’s lab, Department of Marine Biology, University of Haifa
Hebrew editing: Smadar Raban
English editing: Gloria Volohonsky
References:
- A review of how living organisms evolved the ability to produce minerals throughout evolutionary history
- A book surveying how living organisms produce minerals and the biological mechanisms that direct the process
- An article illustrating the link between human CO₂ emissions and the decline in ocean pH
- A paper on the impact of CO₂ levels on coral skeleton formation







