
In a new study, bacteria were genetically engineered to produce paracetamol, the active ingredient in the drugs Tylenol or Acamol. The starting material for the process is not petroleum, but plastic waste. This yields a double environmental benefit: Avoiding the use of petroleum while recycling plastic.
Advertisement
Paracetamol (also known as acetaminophen) is the active ingredient in common pain-relievers and fever-reducing drugs such as Tylenol, Calpol, Acamol, Panadol and others. Two chemists independently synthesized the drug in the second half of the 19th century: Charles-Frédéric Gerhardt in 1852 and Harmon Northrop Morse in 1878. Paracetamol has been in commercial use since 1955 and is produced synthetically from phenol or nitrobenzene, both typically derived from petroleum.
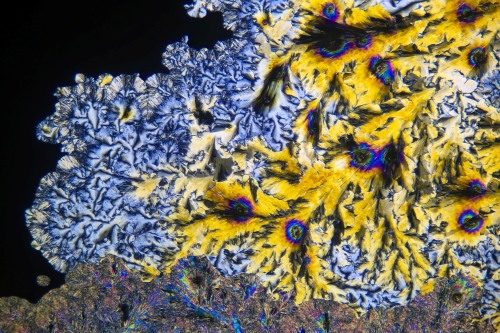
Paracetamol crystals viewed under a microscope. Source: Alexander Klepnev, Wikimedia Commons
In a recent study [1], Stephen Wallace and colleagues at the University of Edinburgh engineered Escherichia coli (E. coli) bacteria, to both recycle a common plastic, PET (polyethylene terephthalate), and to convert the recycling product into the drug paracetamol. This dual process benefits the environment in two ways: it eliminates the need for petroleum for drug production and simultaneously recycles plastic waste.
The chemistry that occurs inside living cells, collectively called metabolism, relies on enzymes. Enzymes are proteins, sometimes with additional components, that catalyze chemical reactions under cellular conditions, allowing them to take place in a functionally-relevant time-frame.
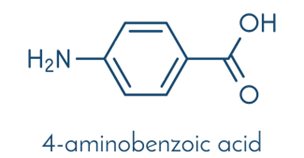
Wallace and colleagues began with E. coli mutants carrying a defect in the gene pabB, which encodes an enzyme in the pathway that produces para-aminobenzoic acid (PABA). PABA is essential for the metabolism of folic-acid, which is crucial for bacteria (and for us). Due to the mutation, the bacteria depend on external PABA supply, so this compound must be supplemented in their growth medium or obtained from another source.
PABA closely resembles an intermediate in paracetamol synthesis—para-aminophenol. The only difference is that PABA contains a carboxyl group (NH₂–C₆H₄–COOH), whereas para-aminophenol contains a hydroxyl group (NH₂–C₆H₄–OH).
In order to obtain paracetamol from PABA, two processes must occur: First, exchange of the carboxyl group from PABA with a hydroxyl group to form para-aminophenol; and then, conversion of the para-aminophenol to paracetamol.
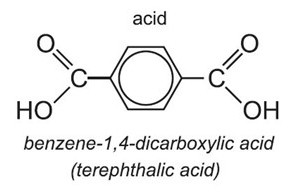
Top: A PET monomer. Note the two carboxyl groups at both ends.
Bottom: Replacing one carboxyl group with an amine yields PABA.
When PET is broken down into its monomers, the main product is an intermediate containing two carboxyl groups. This compound can enter the bacterial cell, where PABA can form non-enzymatically via the Lossen rearrangement, discovered in 1872. This reaction converts one carboxyl group into a primary amine (NH₂) while releasing the carboxyl group. Although the reaction has been observed in the laboratory, it had never been demonstrated in living organisms.
The researchers grew the mutant bacteria without PABA but in the presence of PET. Under various growth conditions they selected mutants in which the Lossen rearrangement provided an alternative route to PABA production. They found that the reaction occurs only in growth medium containing phosphate.
Into these selected bacteria—which convert PET into PABA—they introduced DNA fragments that contain instruction for the production of two enzymes that complete the pathway to paracetamol. The first enzyme, ABH60, originally found in the edible mushroom Agaricus bisporus, converts PABA to para-aminophenol by replacing the carboxyl group with a hydroxyl group. The second enzyme, PANAT, originally found in the bacterium Pseudomonas aeruginosa, acetylates the amine, converting para-aminophenol into paracetamol, whose chemical name is N-acetyl-para-aminophenol..
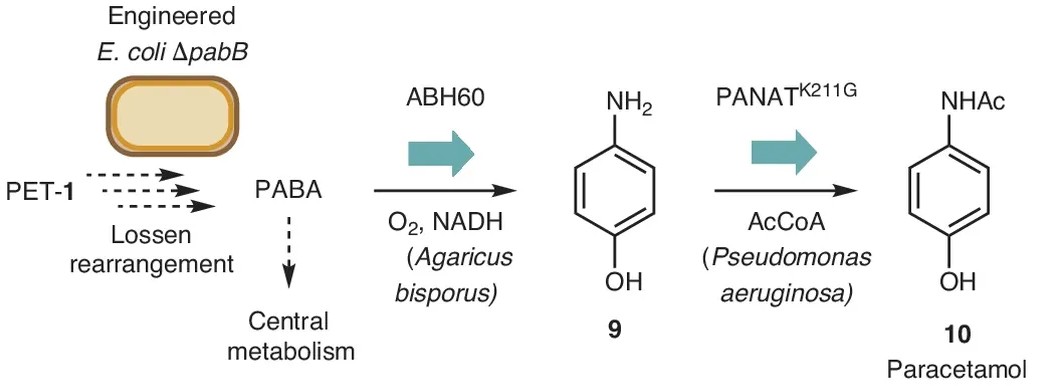
Source: Johnson et al. Nature Chemistry, 2025
This process is a biotechnological and environmental breakthrough. Although it will take some time before bacteria-produced paracetamol reaches commercial use, it seems that the path is not long: the only task left is to adapt the process for efficient large-scale industrial production – a mission for which the knowledge and capability already exist.
Hebrew editing: Smadar Raban
English editing: Elee Shimshoni
References:







